Our experiment used: Pentane: C5H12 with a Boiling point of 309.2K
Introduction:
Through this lab session we are going to divide the class in different groups, and each group will be in charge of a different organic compound, we will observe how their internal pressure varies with and increase of temperature and we will develop a graph using logger pro and its pressure sensor adaptator (to make accurate graphs of our results, naming the respective variables), we are going to apply skills (With the Schlenk tube preparation), we are going to learn how to use a vacuum maker properly and safely, and by comparing our results with the class we will obtain a complete table of results (which will help us develop a conclusion).
There are
4 objectives in this lab session:
1.To improve practical skills
– use of Schlenk tube,
pressure sensors,
vacuum
line.
2.To investigate the structure and properties of one particular chemical.
3.To
investigate the
effect of temperature
on vapour
pressure.
4.To
compare results
with other
groups (with
other chemicals)
and relate them
to “intermolecular forces”.
Equipment:-Vacuum maker(help us have only the gas) - Gas pressure sensor (measure the pressure) -
- Rubber band, Vaseline, Schlenk tube, Stop cog- (Schlenk tube preparation) -
- Adaptor(computer & sensor) - Bath(to test temperature)- Bunsen burner (to alter temperature) -
- Logger pro(measurement programme)-






Procedure:
- We have made a model of Pentane atomic structure.
- We fill our Schlenk tube with Pentane.
- Then we have prepared our Schlenk tube:
- We make the vacuum in the Schlenk tube using the vacuum maker machine
- Then we introduce the Schlenk tube inside of the hot bath of water and connect the adaptor device to our Schlenk and computer.
- We leave it a few minute and it will make function of how changes it pressure depending on its temperature, with its respective table, which measures pressure in kPa depending on temperature.

Conclusion:
Pentane is a compound which has high BP, as we could see in the graph. We can infer that the temperature is a variable that affect significantly the pressure of this molecules from this compound, in this non-flexible container. Has a great BP due to its Van der Waals forces, with a carbon-hydrogen bonding which has an induced dipole. A conclusion from all the table is, that from all the organic compounds, the ones which are completely surrounded by Hydrogen atoms, have significant BP, however the ones which have more lone pairs of Oxygen unequal to the number of bonded Hydrogen atoms have lowest BP, example: Methyl acetate. That is due to the Hydrogen bond intermolecular force, because they are really powerful (10% of a covalent bond), because the lone pairs attract the Hydrogen atoms bonding into this compound, and depending on the proportion of Hydrogen atoms this force increases or decreases.
_________________________________________________________________________________
SOME THEORY FROM INTERMOLECULAR FORCES
*Van der Waals forces are the lowest of intermolecular forces, formed by temporary dipole in one molecule which induce other temporary dipoles to other molecules, they are weak and short lasting (until the molecule charges change).
This type form when randomly moving electrons, some time get in a big a mount in a place of the atom, a temporary dipole occurs which then makes an induced dipole to a near atom, it will last a short time because the electrons will continue moving due their nature.
Larger molecules have this force stronger and due to that a higher boiling point.
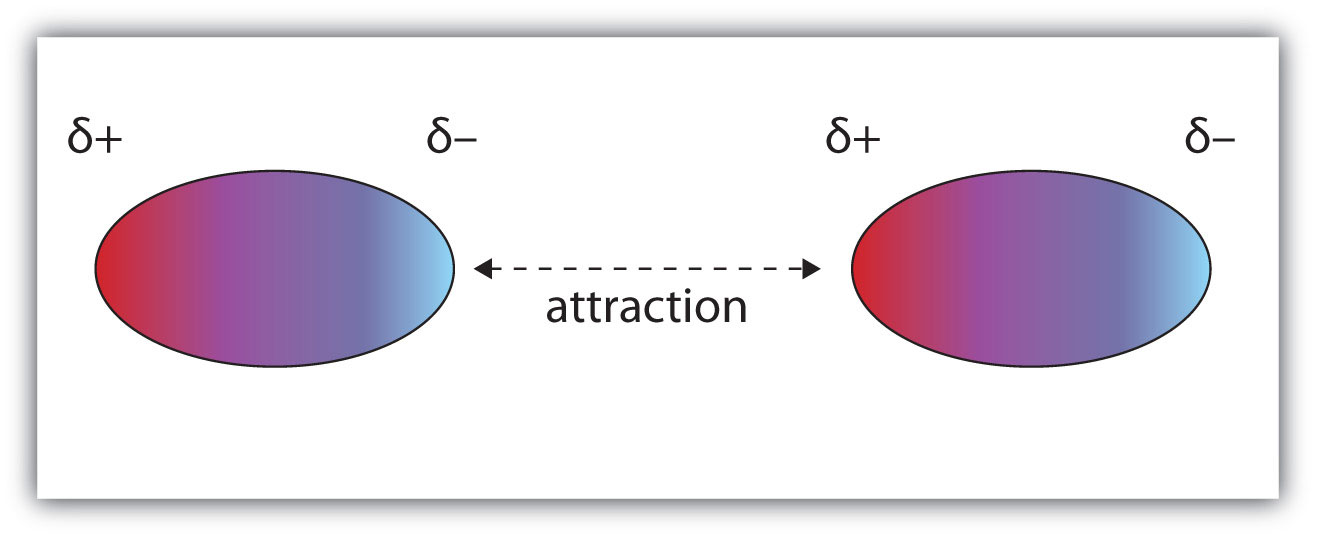
*Dipole to Dipole interaction: is found in polar elements where the dipole is permanent because they have a high electronegative side of the molecule and other low electronegative side. (electronegativity is represented with Delta greek letter) 

Their force will act as in Van Der Waals but longer and stronger, because they are permanent.
If their electronegativity difference is big, their dipole will be larger and with stronger attraction.
*Hydrogen Bondings: strongest intermolecular force caused by the attraction between a lone pair of electrons on a electronegative molecule or particle and an electropositive hydrogen.
Occurs when a Hydrogen is bonded to Fluorine, Oxygen or Nitrogen(those 3 are highly electronegative and a lone pair; a pair of electrons in the outer but not involved in the bonding), the will make hydrogen positive in a permanent dipole, they have the 10% of the strength occurring in covalent bonds.
They have very high boiling points.

References:
- Canning, O. (2014). [online] Retrieved from: http://mrcanning10c.wikispaces.com/Topic+3+-+Intermolecular+forces [Accessed: 25 Feb 2014].- About.com Chemistry. (2014). Organic compounds - list and structures of organic chemical compounds. [online] Retrieved from: http://chemistry.about.com/od/organiccompounds/ [Accessed: 7 Mar 2014].